1. Gastro-oesophageal Reflux Disease (GORD) and Gastritis
 Definition
Definition
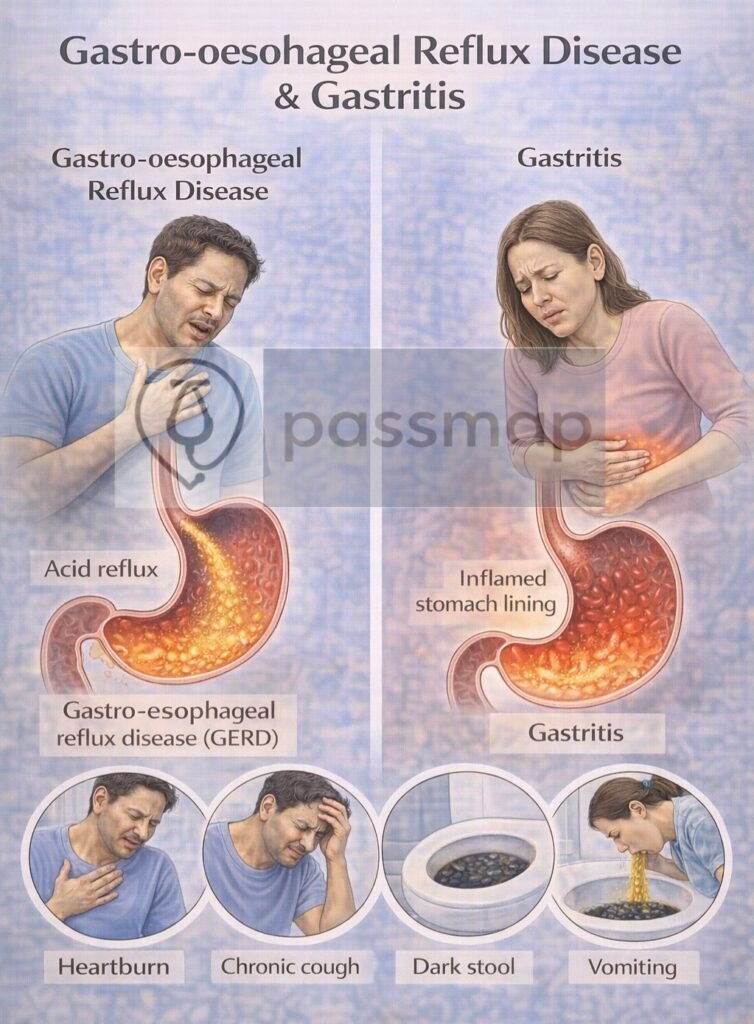
GORD: is a chronic condition where gastric acid refluxes into the oesophagus causing troublesome symptoms (e.g. heartburn) or complications (e.g. oesophagitis).
Gastritis: refers to inflammation of the gastric mucosa (acute or chronic) due to infection, irritants, autoimmune causes, or systemic disease.
🛡️ Aetiology / Risk Factors
| Shared | GORD-specific | Gastritis-specific |
|---|---|---|
| Smoking | Hiatus hernia | Helicobacter pylori infection |
| Alcohol / caffeine / acidic foods | Pregnancy | Autoimmune gastritis (anti-parietal cell / IF Ab) |
| Drugs (NSAIDs, aspirin, steroids, CCBs) | ||
| Elevated BMI (obesity) | Irritants: spicy food | |
| Nicotine | ||
| Associated with GORD |
🤒Clinical Features
GORD – HEARTBURN
- Heartburn – retrosternal burning, worse after meals/lying flat
- Epigastric pain
- Acid taste / regurgitation
- Relieved by antacids
- Troublesome cough (night)
- Bloating
- Unexplained dental erosions
- Repeated sore throat
- Nausea/vomiting
Gastritis – PAINED
- Post-prandial epigastric pain
- Anaemia (chronic blood loss)
- Indigestion / dyspepsia
- Nausea/vomiting
- Early satiety / bloating
- Dark stools (melaena) in haemorrhagic cases
 OGD Referral Criteria
OGD Referral Criteria
 Urgent — 2-Week Wait (ALARM55)
Urgent — 2-Week Wait (ALARM55)
Urgent referral for OGD (oesophagogastroduodenoscopy) (2WW) if:
- Anaemia (iron deficiency)
- Loss of weight (unintentional)
- Anorexia
- Recent onset dysphagia
- Melaena / haematemesis
- 55 years or older with new symptoms
2️⃣ Non-Urgent OGD — Barrett’s Risk Assessment
Consider non-urgent endoscopy for patients with GORD symptoms + multiple Barrett’s risk factors, even without red flags:
Chronic GORD symptoms (>5 years)
Age ≥50
Male sex
White ethnicity
Central obesity (waist circumference / BMI ↑)
Smoking history
First-degree relative with Barrett’s oesophagus or oesophageal adenocarcinoma
Purpose: Detect and confirm Barrett’s early for surveillance.
 Investigations
Investigations
| Test | Indication | Notes |
|---|---|---|
| Clinical diagnosis | Typical symptoms, no red flags | Empirical treatment appropriate |
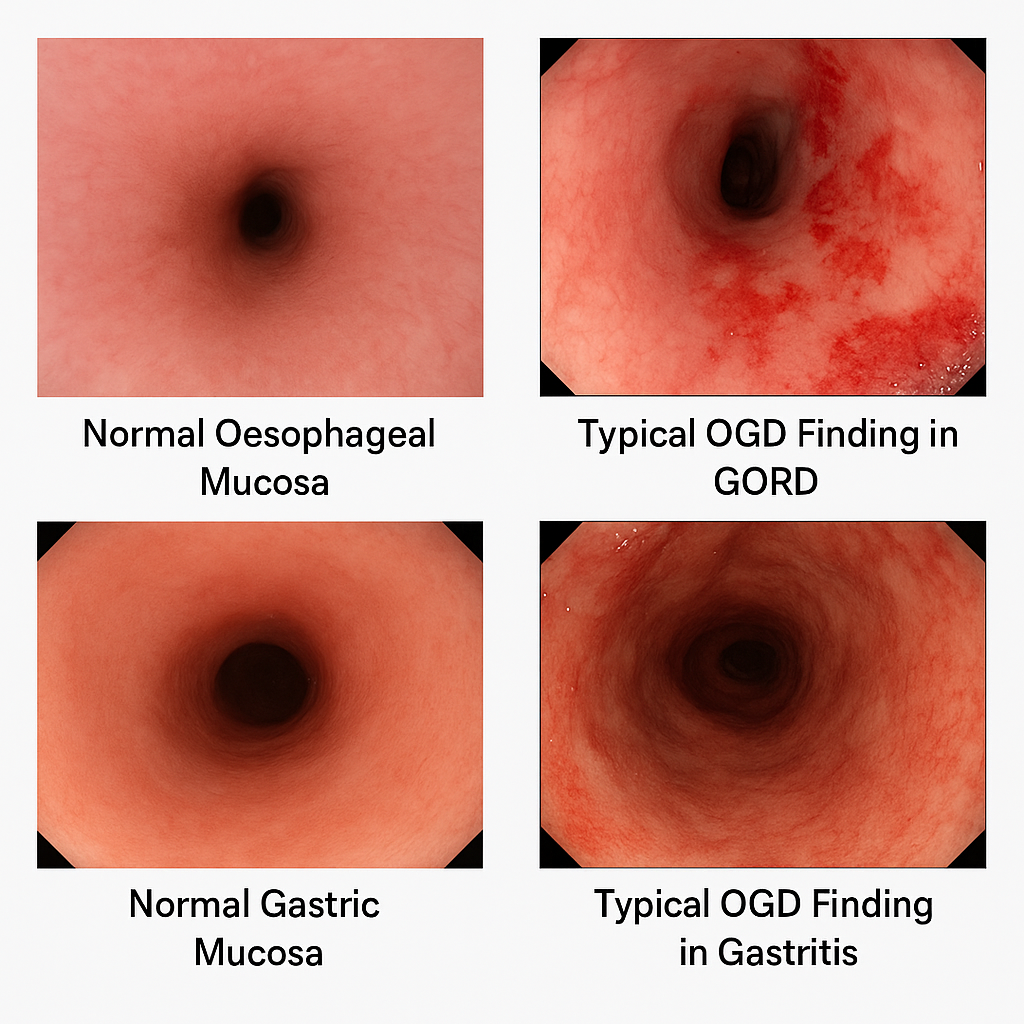
| OGD | ≥55y + new symptoms • Alarm features • Poor treatment response | Biopsy if Barrett’s suspected |
| pH monitoring / manometry | Diagnosis uncertain after OGD | Useful for surgical planning |
| H. pylori testing | See treatment ladder | Stop PPI ≥2 weeks prior |

 Initial Assessment
Initial Assessment
Exclude red flags (↑ → urgent OGD)
Assess NSAID, alcohol, smoking use
Consider overlap with PUD or functional dyspepsia
 First-line in No Red Flags
First-line in No Red Flags
Lifestyle changes (see management)
Empirical PPI trial – omeprazole 20 mg OD for 4 weeks

 H. pylori Testing — When to Test?
H. pylori Testing — When to Test?
 Test for H. pylori if:
Test for H. pylori if:
- Un-investigated dyspepsia (especially <55 years and no red flags)
- Symptoms persist after PPI trial
- Previous history of gastric/duodenal ulcer or gastritis
- Planned long-term NSAID use, especially in patients >45 years
- Known iron-deficiency anaemia, or ITP/B12 deficiency
 Do not routinely test:
Do not routinely test:
Asymptomatic, no ulcer history or risk factors
During/within 2 weeks of PPI use
If alarm symptoms present → refer OGD
 PARA Tip:
PARA Tip:
PPIs must be stopped ≥2 weeks before urea breath test or stool antigen — common exam trap.

 H. pylori Testing — Which Test to Use?
H. pylori Testing — Which Test to Use?| Test | When to Use | Notes |
|---|---|---|
| Urea Breath Test | 1st-line | Stop PPI ≥2 wks before |
| Stool Antigen Test | 1st-line | Preferred in primary care |
| Serology | Avoid | Cannot distinguish past vs active infection |
| OGD + biopsy | Red flags / failure | Also rules out malignancy & ulcers |

 Follow-Up
Follow-Up
- Confirm eradication 4 weeks after completing triple therapy (if given) using urea breath test or stool antigen — not serology.
 Summary for PARA:
Summary for PARA:
Test H. pylori in persistent dyspepsia without alarm features. Use stool antigen or breath test (PPIs stopped 2 weeks prior). Do not test if alarm symptoms present — refer for endoscopy.
 Management —
Management —
 Lifestyle & PRN Relief
Lifestyle & PRN Relief
Weight loss, smoking/alcohol cessation, avoid trigger foods/drinks, smaller frequent meals, avoid lying after eating, raise head of bed.
PRN antacids/alginates (e.g. Gaviscon Advance).
 Standard PPI Trial
Standard PPI Trial
Omeprazole 20 mg OD × 4–8 weeks.
PHE advice: In uncomplicated dyspepsia, test for H. pylori after PPI trial (low UK prevalence <15%).
If improved → step down to lowest effective dose/on-demand.
Breakthrough Symptoms
Check adherence & lifestyle.
Offer PRN antacids between PPI doses.
If persistent → double PPI dose or trial alternative PPI.
 Step-Up or Alternative
Step-Up or Alternative
Double PPI dose (e.g. omeprazole 20 mg BD).
Switch to alternative PPI.
Add H2RA (e.g. famotidine) if PPI not tolerated.
 H. pylori Eradication (if positive)
H. pylori Eradication (if positive)
Triple therapy: PPI + amoxicillin + clarithromycin/metronidazole × 7 days.
Confirm eradication after 4 weeks (off PPI ≥2 weeks).
 Refractory / Severe
Refractory / Severe
OGD to rule out malignancy, Barrett’s, ulcers.
Consider surgical fundoplication for GORD if severe & PPI-resistant.
Manage autoimmune gastritis (lifelong B12 if pernicious anaemia).
 Complications
Complications
GORD – Mnemonic: BEACH
Barrett’s oesophagus
Esophagitis
Anaemia
Carcinoma (adenocarcinoma risk)
Haematemesis
Gastritis – Mnemonic: BAGS
- Bleeding
- Anaemia
- Gastric atrophy
- Stomach cancer
🔎 Barrett’s Oesophagus (Key Complication)
Metaplasia: squamous → columnar epithelium
Risk of oesophageal adenocarcinoma
OGD surveillance with biopsies
Dysplasia: consider RFA or endoscopic resection
🔎 Last updated in line with:
NICE NG1 (Gastro-oesophageal reflux disease in children and young people: diagnosis and management) – Published Jan 2015 • Last updated Oct 2019
NICE CKS (Dyspepsia – acute and chronic) – Published May 2010 • Last updated Apr 2023
- PARA-aligned, reviewed February 2026
PASSMAP ensures all content is NICE-aligned and reviewed for Physician Associate Registration Assessment (PARA) success
Educational platform. Not medical advice.
